Acid rain
Acid rain is the kind of precipitation that contains larger amounts of acid than normal. Rainwater is usually slightly acidic, with pH level between 5 and 6. Water that evaporates from earth is neutral (pH 7) and it becomes weak acid when mixed with carbon dioxide in the atmosphere. Acid rain contains more pH than ordinary. This is caused by the presence of air pollutants, like sulfur dioxide and nitrogen oxides. They produce acids if combined with water. Acid rain is considered as the wet deposits of air pollutants, where it's combined with moisture before falling into the ground. While air pollutants that fall without combining with moisture is called dry deposits.
Acid rain can occur naturally, from the volcanic eruptions. However we are also causing this, from the emission of vehicles and of industrial plants that include the burning of fossil fuels. If we continue to increase rate of air pollution, we are increasing the risk of acid rain to happen.
What's the impact of acid rain?
- Deteriorates building that is made of rock
- Acidification of soil and lakes
- Separation of poisonous minerals such as aluminum and mercury from the surrounding ground, increasing the risk of contamination to lakes/water sources
- Deteriorates trees and forests.
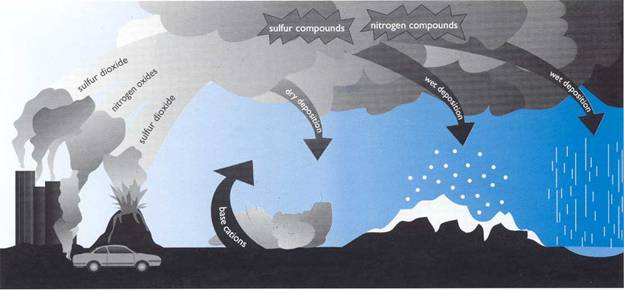
Mechanism of acid rain
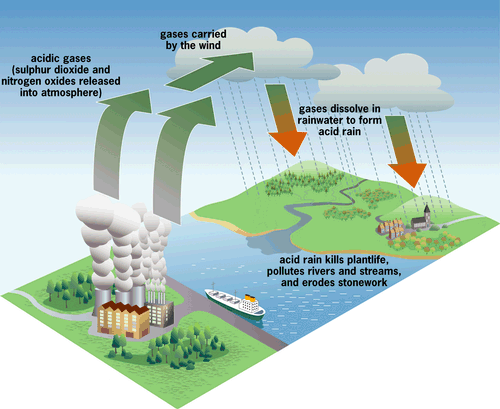
Acid rain |


