Ozone depletion
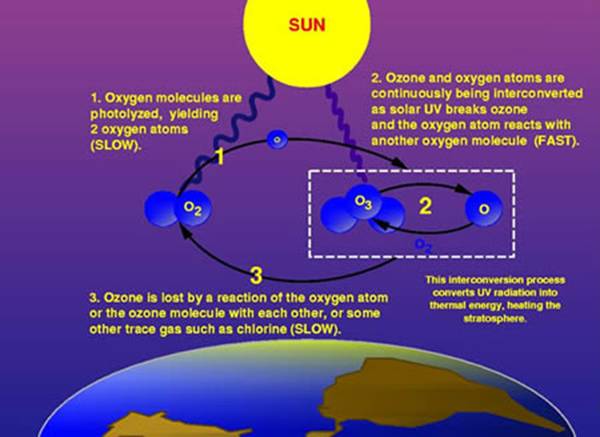
Ozone layer is a protective layer in our atmosphere (O3, three oxygen atoms). It's about 19 to 30 km in distance from the Earth surface. It plays an important role of blocking ultraviolet (UV) rays that come from the sun, which, if there was no ozone layer ever, cancer would dominate and even no life would be in this world! The concentration of the layer is usually under 10 parts ozone per million. The ozone layer is made up by the action of sunlight to oxygen, and the amount is stabled by the existence of nitrogen.
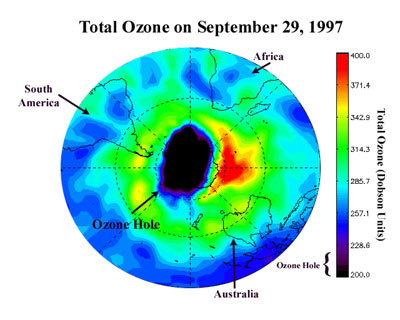
In today's trends there is a noticeable depletion of the ozone layer. It's popularly known since 1970 that a substance called CFC (chlorofluorocarbon) is threatening the layer. This substance is usually contained in refrigerators, coolants, and aerosol sprays. When we use much of those things (which contain CFC), we are continually depleting our Earth's ozone layer. However, most of the latest products today do not contain CFC anymore. Some other substances, like bromine halocarbons and nitrous oxides are also possible threats.
|
|
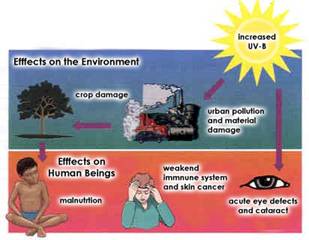
The effects of ozone layer depletion are:
-
More ultraviolet rays come to Earth (this could make the Earth just like a cooking oven)
-
More heat, thus increasing the risk of global warming
How CFC depletes the ozone layer?
-
CFC molecule, consisting of one atom for each fluorine and carbon and 3 chlorine atoms, is hit by the UV rays.
-
One chlorine atom breaks apart. It will hit an ozone (O3) and takes one oxygen atom away to create chlorine monoxide, thus leaving one oxygen molecule (O2).
-
Another oxygen atom breaks the chlorine monoxide and takes the oxygen atom away, leaving one chlorine atom, leaving no ozone molecule. Process repeats.
|



